Opioid Agonist Therapy (OAT)
Methadone Oral Concentrates for Opioid Agonist Therapy (OAT) in Saskatchewan
The Saskatchewan Drug Plan and Extended Benefits Branch (SK DPEBB) and Non-Insured Health Benefits (NIHB) formularies list SIX methadone oral concentrates (10 mg/mL) for OAT, including three listed generic products.
Methadone 10 mg/mL Oral Concentrates According to Interchangeability on the SK DPEBB Formulary
| Brand Name Product | Interchangeable Product(s) |
| Metadol-D® | No interchangeable products |
| Methadose™ Sugar-Free | Odan-Methadone Sugar-Free |
| JAMP Methadone Sugar-Free | |
| Methadose™ Cherry Flavoured | Odan-Methadone Cherry Flavoured |
Compounded methadone is not a benefit of the SK DPEBB formulary. In exceptional circumstances, DPEBB will consider, on a case-by-case basis, compounded methadone as Exception Drug Status (EDS).
Comparison of Methadone
Products for OAT in SK

Historical Context
The information below was developed to support health care providers transition people who use methadone from compounded methadone to commercially available methadone. Now that the transition is complete, some of this information is no longer relevant but has been kept for historical context. Much of the information remains relevant in terms of recommendations and rationale, prescription standards, and supporting people using methadone. The information has been updated to include all available methadone oral concentrate products for OAT.
- Background & Reasons for the Change
- Methadone hydrochloride is used for pain management and/or as opioid agonist therapy (OAT) for opioid use disorder (OUD).
- When used for OUD, pharmacists in SK have traditionally compounded a stock solution from methadone powder and water, which was stored in the fridge. The prescribed dose of this solution was diluted with a suitable diluent (a liquid vehicle, such as Orange Tang™).
- On September 1, 2022, three commercially available methadone 10 mg/mL oral concentrates were added as listed benefits on the SK DPEBB Formulary. In November 2023, three additional methadone 10 mg/mL oral concentrates were listed on the SK DPEBB Formulary. All of these products are on the Non-Insured Health Benefits (NIHB) Drug Benefit List.
- Compounded methadone is only approved by the SK DPEBB in exceptional circumstances on a case-by-case basis.
Reasons for the change include:
- Commercially available products:
- are consistently prepared and standardized products resulting in enhanced safety and quality control,
- are recommended by the Institute for Safe Medication Practices Canada (ISMP Canada),
- do not require refrigeration1,2 (before dilution),
- may be associated with less wastage (due to the 14-day beyond-use date of the compounded stock solution if not preserved), and,
- must be used rather than compounded solutions, per Health Canada Policy, whenever they are readily available3.
- Regulatory changes to compounding standards in SK were implemented on August 31, 2022, which seek to ensure the safety of both patients and the personnel involved in compounding, as well as establish additional quality controls. After this date, numerous pharmacies (i.e., those who have not declared as being Level B or C) are not authorized to compound complex non-sterile compounds, such as methadone.
- By removing the safety risks associated with compounding, and simplifying the preparation process for methadone, more pharmacies in Saskatchewan may begin dispensing OAT, reducing barriers to access for people taking methadone.
- All other jurisdictions in Canada have already transitioned away from using compounded methadone and are using the commercially available products as the preferred products.
- Aligning the availability of products across provinces and territories, allows people taking methadone to maintain consistency of the product when they move between jurisdictions.
- The reasons for the change are believed to all be important and, ultimately, to be in the best interest of people taking methadone for improving safety.
- Product Availability
- As shown in Table 1, four brands of methadone oral concentrates (i.e., Metadol®, Methadose™, Odan-Methadone, and JAMP Methadone) and six specific formulations (i.e., Metadol-D®, Methadose™ Sugar-Free, Methadose™ Cherry Flavoured, Odan-Methadone Sugar-Free, Odan-Methadone Cherry Flavoured, and JAMP Methadone Sugar-Free) are listed benefits of the SK DPEBB for use as OAT. These are also on the NIHB Drug Benefit list.
Table 1: Specifying the Methadone Brand and Formulation
Brand Formulation Metadol® -D Methadose™ Sugar-Free Odan-Methadone JAMP Methadone Methadose™ Cherry Flavoured Odan-Methadone - In accordance with the SCPP OAT Standards, Methadose™ Cherry Flavoured use should be limited to patient request due to the risk of contributing to destabilization.4
- To reduce the chance of dosing errors, pharmacies are required to dispense only the 10 mg/mL commercially available oral concentrates for OAT.
- It is important to recognize there is risk of potential error when pharmacies carry different products of the same medicinal ingredient. There is also risk to people taking methadone when they are not offered choice regarding their medication treatment.
- Clinicians are encouraged to actively engage with people taking methadone and other providers involved in their care, to offer methadone-related information and recommendations (see Product Selection), while also not creating barriers to access to specific brands and formulations.
- Product Selection
Considerations to support the decision regarding which product to select should include factors such as relative effect, safety, formulation properties, convenience, cost, as well as patient perceptions and preferences.
Please see Commercially Available Methadone for Opioid Agonist Therapy (OAT): Characteristics of Products Available for Use in Saskatchewan here, for an overview of the product characteristics.
medSask/USask CPE Recommendations
- Providers should collaborate with people taking methadone to discuss options. Engaging people in their own health care decision-making can improve the outcomes associated with change.
- There are unfortunately no high-quality studies to direct the treatment choice at this time. In the absence of robust clinical trials to draw from, lower quality observational trials and anecdotal information provides the only information on which to base decision-making at this time. Lived experience is an important consideration.
- Metadol-D® may be preferred by people taking methadone and clinicians and is recommended by medSask/USask CPE as a first choice.
- There are potentially serious safety concerns associated with Methadose™ related to lack of effect which have been anecdotally reported by some people taking methadone and clinicians in Canada. Given this risk, even though not yet clearly established in the scientific literature, alongside reports that Metadol-D® has been preferred by people and clinicians with experience, it may be prudent to use this product preferentially until further evidence becomes available.
- From a logistical perspective, recommending this as a single methadone product may also improve safety, by simplifying processes in the steps relating to prescribing and dispensing, as well as supporting people taking methadone who may require "guest dosing".
- Methadose™ Cherry Flavoured is not recommended as a first choice in SK. However, it may be appropriate to continue this product if a person has already been stabilized on it and wishes to continue taking it. Use should be limited to prescriptions written on the specific request of a person taking methadone, after ensuring informed consent is provided and documented.4
medSask/USask CPE recommendations are based on these considerations:
Relative Effect and Safety
- In 2020, Health Canada released a risk communication, which identified a potential risk of lack of effect (i.e., causing the person to experience opioid withdrawal symptoms) and clinical destabilization when switching between different methadone products.5 Reasons for this difference in effect are unclear. Health Canada did not indicate a specific brand or formulation of concern.
- The Methadose™ Cherry Flavoured brand and formulation has been described in the literature as causing lack of effect and clinical destabilization (e.g., people taking methadone becoming “dope sick” or experiencing “withdrawals”), which can be a serious safety concern. (See Exploring the Reports of Clinical Destabilization for more information.)
- For this reason, SCPP OAT Standards indicate use should be limited to patient request.4
- It is unknown whether the lack of effect associated with the Methadose™ Cherry Flavoured formulation is similar with the Sugar-Free formulation.
- The literature does not report on the Sugar-Free formulation and no randomized controlled trials or head-to-head analyses have been performed to compare the formulations.6 Anecdotal reports suggest some people taking the Methadose™ Sugar-Free formulation have experienced lack of effect and clinical destabilization.
- Comparatively, in a Commentary written by clinicians in BC, it was reported that, “Fortunately, offering Metadol-D as an alternative to Methadose appears to be greatly beneficial.”7
- It may also be safer if clinicians preferentially recommend, prescribe, and dispense a single methadone product, to further reduce the chance of confusion and error in the prescribing and dispensing processes.
Formulation Properties
- Metadol-D®, Methadose™ Sugar-Free, and Odan-Methadone Sugar-Free are similar in their taste and appearance (flavourless with no sweetener and colourless).
- JAMP Methadone Sugar-Free is sweetened with sorbitol and is blue in colour.
- Methadose™ Cherry Flavoured and Odan-Methadone Cherry Flavoured have a distinct taste, colour, and thickness, which may reduce palatability.
- Metadol-D® contains dextrose.
- Communication with the manufacturer indicates that Metadol-D® contains dextrose in a concentration of 125 mg/mL (Paladin Labs, personal communication, July 6, 2022). This concentration is low enough that it is unlikely to have clinical significance for people, such as those with diabetes or fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) intolerance. E.g., a Metadol-D® 100 mg PO daily dose = 10 mL = 1.25 g of dextrose = 4.25 calories.
Interchangeability
- There are no generic products interchangeable with Metadol-D®.
- People using Methadose™ Sugar-Free or Methadose™ Cherry Flavoured may be switched to the appropriate generic product should they choose or in the event of product procurement issues.
- Ensure they are aware of differences between products. For example, unlike Methadose™ Sugar-Free, JAMP Methadone Sugar-Free is blue in colour.
- It is prudent to monitor patients during the switch. See Prescription Information → Monitoring.
Convenience
- All products should initially be prescribed at the same dose and interval that the person previously received.
- If particular products do not last for twenty-four hours (e.g., “wear off” early), as has been described in the literature with the Methadose™ Cherry Flavoured formulation, this may lead to clinical destabilization. Destabilization can result in the need for the person to make more frequent healthcare visits, lose authorization for take-home doses, and/or require split dosing at the pharmacy.
- Additionally, if a single product is used preferentially in SK, this will support people taking the product who might require “guest dosing” from a different pharmacy due to unexpected circumstances (e.g., travelling for a funeral, family emergency, rapidly changing living situations).
Cost
- There may be price differences among the methadone oral concentrate products.
- Practitioners should consult current listing prices in circumstances when the cost becomes significant for people taking methadone (e.g., those not receiving provincial or federal drug plan benefits and without third party insurance).
- Saskatchewan residents with a valid Saskatchewan Health coverage may be eligible for DPEBB benefits such as the Special Support Program. Pharmacy professionals should ensure patients using methadone have Special Support coverage in place and assist them in the application process if necessary. There are other benefits for which patients may be eligible; pharmacy professionals are encouraged to make patients aware of these programs and refer them to the applicable agency.
Perspectives and Preferences of People Taking Methadone Alongside Clinical Experience
- Patient perspectives and experiences, particularly for structurally vulnerable individuals, are at times under-represented in the medical literature.
- Clinicians should be aware that the Methadose™ brand as a whole has been associated with negative experiences and public perception.
- Board members from an advocacy group comprised of people with lived experience in British Columbia (e.g., the British Columbia Association for People on Opioid Maintenance) have advised to, “Avoid Methadose. Choose Metadol-D.” (L. Shaver, personal communication, June 21, 2022 and G. Mullins, personal communication, July 7, 2022).
- A podcast by people with lived experience has supported the use of Metadol-D®, reporting that it, “… seems to work better” (than the Methadose™ Cherry Flavoured) and, “…doesn’t have a dose-holding problem.”8,9
- Regarding the reports of Methadose™ Cherry Flavoured wearing off early, people have been quoted as describing it as, “It doesn’t haven legs!”9
- Also anecdotally, some have suggested that the lack of documented reports in Canada outside of BC may be related to having fewer organized activist groups to speak to these issues.
- An electronic mailing list, META:PHI, composed of clinicians involved in addiction medicine, has provided a forum for some comments regarding experiences with the methadone products.
- Some clinicians in Canada have described having patients experience withdrawal, particularly after switching to a Methadose™ product (reported with both the Cherry Flavoured and the Sugar-Free formulations), often alongside a preference for Metadol-D®.
- Patients may prefer Metadol-D® because it has been reported more favourably by some people taking methadone and clinicians in other parts of the country, in terms of relative effect and safety, and because it has a comparable appearance and taste to the compounded methadone.
- Characteristics of Commercially Available Methadone 10 mg/mL Oral Concentrate
Comparisons of the methadone concentrate products available for OAT in Saskatchewan can be found here.
- Prescription Information
- When converting from one methadone product to another, continue the same dose.
- Prescriptions must be written in milligrams (mg).
- Since commercially available methadone products are not interchangeable, the product selected must be prescribed by brand and formulation.
- If selecting Metadol-D®, ensure the prescription includes the “-D” (plain Metadol® is for pain only).
- If selecting Methadose™, ensure the prescription specifies the Sugar-Free formulation.
- Odan-Methadone Sugar-Free and JAMP Methadone Sugar-Free are interchangeable with this product.
- Methadose™ Cherry Flavoured is not recommended as a first choice.
- If selected, Odan-Methadone Cherry Flavoured is interchangeable.
- Under the Section 56 Exemption, pharmacists are not authorized to prescribe for the change of prescription. A verbal order may only be accepted after every effort has been made to obtain a written or e-prescription.
- If the methadone formulation is being switched (i.e., switching to a product that is not interchangeable):
- It is recommended that prescriptions are coordinated to start the new formulation on a day when the person receives a witnessed dose. This will allow for the pharmacist to discuss any concerns or questions the person has at the time of taking their first dose. It will also allow for the subsequent take-home doses (if applicable) to be prepared and dispensed as a single formulation.
- Plan to start the new formulation early in the week (e.g., Monday or Tuesday), whenever possible, to enhance access to providers for follow up or changes if needed.
- While it is not required, changes to take-home doses may be desired following a methadone formulation switch to facilitate enhanced contact, monitoring, and communication with a pharmacist. This may be particularly relevant for those people who have very recently entered the maintenance phase of taking methadone.
- If there is particular concern, anxiety, or distress associated with making a change, people taking methadone may benefit from proactively having a prescription for pharmacological opioid withdrawal adjuncts logged on file at the pharmacy. This may include medications such as clonidine, loperamide, and non-steroidal anti-inflammatory drugs (note: see RxFiles “Prescription for Managing Opioid Withdrawal” available through SHIRP).
- If opioid withdrawal occurs a few days after the methadone switch, this provides the person taking methadone an option to help manage their symptoms until the methadone prescription can be reassessed by the prescriber (See Responding to Clinical Destabilization).
Sample Methadone Prescription - Note brand and formulation are specified
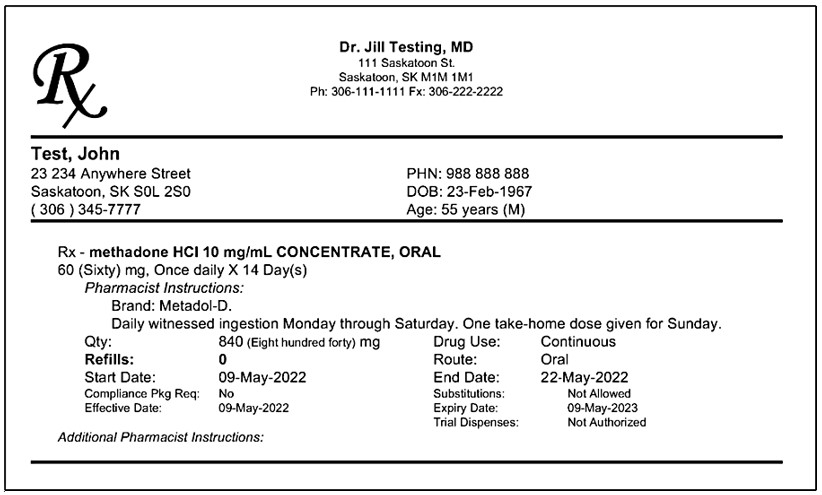
Monitoring
- Monitor people taking methadone for lack of effectiveness and safety concerns regarding their regimen following transitions between products. See the SCPP OAT Standards, the College of Physicians and Surgeons of Saskatchewan (CPSS) OAT Standards and Guidelines, and specific product monographs for monitoring parameters.1,2,4
- Some people taking methadone in Canada have reported severe withdrawal symptoms (lack of effect of the methadone) typically within a few days following a switch in methadone product (the literature specifically describes this happening with the Methadose™ Cherry Flavoured product).
- Symptoms of lack of effect reported include sweating, insomnia, tremor, agitation, nausea/vomiting/diarrhea (i.e., feeling more “dope sick” or “withdrawals”), increase in pain, increase in cravings and/or feelings of needing to supplement with other opioids.
- If symptoms develop, consider using COWS or SOWS scoring for monitoring and assisting with the establishment of symptom severity.
- Ensure any concerns are communicated to the prescriber. It is important for people to know they are supported through this transition by their team of providers.
- Responding to Clinical Destabilization
- There is a risk of destabilization with any change of OAT.
- Serious destabilization has been associated with the Methadose™ Cherry Flavoured product, though high-quality evidence to establish causality is not available. The best available evidence that speaks to this association includes a qualitative study (N=34), a survey (N=405), a cohort study (N=331), and anecdotal reports.7,10-12 All published reports have come out of British Columbia, and therefore the severity or extent of impact related to this change in other jurisdictions is also not well established.
- It is unknown if there is a difference in destabilization risk between formulations.
- Health Canada has identified a potential risk of lack of effect (i.e., causing the person to experience opioid withdrawal symptoms) and clinical destabilization when switching between different methadone products.5 Health Canada did not indicate a specific brand or formulation of concern.
- Validate, reassure, and address the concerns of people who report a lack of effect in the first few days after switching to a new methadone formulation.
- If withdrawal or destabilization occurs, the prescriber is encouraged to consider one of the following modifications to the methadone prescription as early as possible:
- Switch to a different methadone brand and formulation (i.e., from Methadose™ Sugar-Free to Metadol-D®).
- Divide the methadone dose, such as to split the total daily dose to twice daily (e.g., methadone 100 mg PO daily could be divided to 50 mg PO BID). This approach may not be feasible if take-home doses are deemed unsafe in the clinical circumstance and/or the person is unable to attend the pharmacy twice in a day.
- Trial an increase in the methadone dose (e.g., by 5-10 mg, no more often than every 5-7 days). There is concern that if the medication is wearing off in advance of 24 hours, a dose increase may not be sufficient to overcome this effect.
- If clinical stability cannot be achieved with these methadone adjustments, or if the person wishes to explore a different option, consider whether an alternate form of OAT, such as a buprenorphine product, might be appropriate (note: buprenorphine may be less effective than methadone for people who use high opioid doses, e.g., large amounts of fentanyl regularly). Consult with an expert in prescribing OAT and/or addiction medicine as required.
- All people experiencing withdrawal and/or destabilization will require closer follow up and possibly reassessment of the take-home dose authorization (if applicable), until re-establishment of clinical stability on OAT.
- Any changes in clinical response associated with a commercially available product, including withdrawal symptoms soon after the transition, or other serious or unexpected adverse reactions in people taking methadone should be reported to Health Canada to support ongoing monitoring of the safety of commercially available products.5
- There is a risk of destabilization with any change of OAT.
- Exploring the Reports of Clinical Destabilization
- High-quality clinical evidence to establish causality is not available; however, it is important to take reports of changes in effect seriously (i.e., getting “dope sick” or having “withdrawals”) since clinical destabilization can be life-threatening. Lack of effect may result in people “topping up” with opioids from the illicit drug supply which are often contaminated and potentially lethal.
- In British Columbia, where this was primarily reported starting in the first few days after people were switched from compounded to the commercially available methadone in 2014, the lack of effect leading to opioid withdrawal was related to the Methadose™ Cherry Flavoured concentrate.
- Researchers in British Columbia published a study in 2016, in which 405 people who had been switched to the Methadose™ Cherry Flavoured product were surveyed. Of the respondents, 56% (202/358) reported feeling more dope sick and 54% (193/358) reported more pain after the formulation change. Also following the switch, 50% (181/360) of people surveyed reported supplementing with other opioids, and 33% (107/320) had an increase in their methadone dose.10
- Another observational study quoted a participant as saying, regarding the switch to the Methadose™ Cherry Flavoured concentrate, “It’s not agreeing with my body. I can feel a little bit of the effects of it [alleviation of withdrawal symptoms]. Other than that… the pain seems to be a lot more than it was when I was taking the regular methadone. I still don’t understand this [obscenity], but I don’t think I really wanna be on it [Methadose®]. In the middle of the night, I feel nauseated, whereas before I was always fine…I get my methadone at 6:30 [am] and by about eight or nine o’clock at night, my body aches a lot more.”12
- The exact reason for the difference in effect is not known. Theories include:
- Differences in viscosity and volume - this product is thicker and does not require dilution. As such, the smaller and “stickier” amount may adhere more readily to the dispensing container resulting in a partial loss of the more concentrated dose.
- Potentially different ratio of enantiomers – methadone is a chiral molecule. Given that its R- and S-enantiomers may not fit into the receptors in the same way, a change in their relative amounts could cause a change in effect.
- Psychological effects - the smaller (undiluted) dispensed volume, feelings of loss of control secondary to imposed changes, and a disagreeable taste/consistency may all contribute to lack of effect of the medication.
- In consideration of the observational and anecdotal reports, Methadose™ Cherry Flavoured is not recommended as a first choice when transitioning people to a new methadone formulation at this time.
- Also, since the differences between the Sugar-Free and Cherry Flavoured formulations are not known, and in keeping with what is recommended by some people with lived experience, people are encouraged to consider using Metadol-D preferentially at this time.
- Any change can be difficult, and it is important for providers to follow up with people who are making the transition. People who feel supported and are well-informed about this change along with the potential implications will be more likely to return to their prescriber early on to communicate any concerns.
- Tips for Communication with People Changing Methadone Formulations
- Remember to treat each person as an individual, with respect and dignity, and acknowledge that any changes directly impact their life.
- Emphasize the importance of continuity of care and encourage the person to stay with the same methadone prescriber and pharmacist, whenever possible, through the transition.
- Commit to helping each person create a treatment plan that meets their own goals, values, and preferences.
- It may be helpful to explain the difference between products, reiterate that diversion risks remain, and that people must safely store their medication and not share or sell it to others.
- Invite people to discuss any medication concerns early on with their care provider - including concerns before and after the change in methadone formulation – and offer reassurance that steps will be taken to partner with them to maintain and/or regain clinical stability after the formulation change.
- References
1. Metadol-D Product Monograph. [cited May 3, 2022]. Paladin Labs Inc. Last revised February 25, 2021. Available from: https://www.paladin-labs.com/our_products/Metadol-D_En.pdf.
2. Methadose Product Monograph. [cited May 3, 2022]. Mallinckrodt Canada ULC. Last revised August 22, 2018. Available from: https://pdf.hres.ca/dpd_pm/00047060.PDF.
3. Policy on Manufacturing and Compounding Drug Products in Canada (POL-0051). Health Canada. Issued January 26, 2009. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/good-manufacturing-practices/guidance-documents/policy-manufacturing-compounding-drug-products.html.
4. Opioid Agonist Therapy (OAT) Standards. Saskatchewan College of Pharmacy Professionals. Last revised October 2020. Available from: https://www.saskpharm.ca/document/5871/REF_OAT_Standards.pdf.
5. Health Professional Risk Communication. Methadone treatment of opioid dependence and potential risk of lack of effect when switching between different products. Health Canada. July 17, 2020. Available from: https://recalls-rappels.canada.ca/en/alert-recall/methadone-treatment-opioid-dependence-and-potential-risk-lack-effect-when-switching.
6. Metadol Liquid, Methadose, and Compounded Methadone in Patients Using Opioid Agonist Therapy: Comparative Clinical Effectiveness and Guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2018 Apr 12. (CADTH rapid response report: summary of abstracts). Available from: https://www.cadth.ca/sites/default/files/pdf/htis/2018/RB1208%20OAT%20Methadose%20Final.pdf.
7. Raski M, Sutherland C, Brar R. From methadone to Methadose: Lessons learned from methadone formulation change in British Columbia. Can Fam Physician. November 2020;66(11):797-98.
8. Mullins, G (Host). Change Intolerance (Episode 2). [Audio Podcast Episode]. In Crackdown. British Columbia. Feb 27, 2019. Available from: https://www.crackdownpod.com/episodes/c698nin092b486ji2hycfuf9gpo15v.
9. Mullins, G (Host). Change Intolerance Part 2 (Episode 9). [Audio Podcast Episode]. In Crackdown. British Columbia. Oct 31, 2019. Available from: https://www.crackdownpod.com/episodes/pmbyiswxmpr3hxnbee3qjt1nz50ylm.
10. Greer AM, Hu S, Amlani A, et al. Patient Perspectives on Methadone Formulation Change in British Columbia, Canada: Outcomes of a Provincial Survey. Subst Abuse Treat Prev Policy. 2016;11(3). https://doi.org/10.1186/s13011-016-0048-3.
11. Socias ME, Wood E, McNeil R, et al. Unintended impacts of regulatory changes to British Columbia Methadone Maintenance Program on addiction and HIV-related outcomes: an interrupted time series analysis. Int J Drug Policy. 2017;45:1-8. https://doi.org/10.1016/j.drugpo.2017.03.008.
12. McNeil R, Kerr T, Anderson S, et al. Negotiating Structural Vulnerability Following Regulatory Changes to a Provincial Methadone Program in Vancouver, Canada: A Qualitative Study. Soc Sci Med. 2015;133:168-176. https://doi.org/10.1016/j.socscimed.2015.04.008.
Last Updated: 31 Oct 2023