Pharmacists and primary care providers play a key role in supporting patients transitioning to a biosimilar. This webpage will give you links to familiarize yourself with biologic drugs, biosimilars, and the Saskatchewan Biosimilars Initiative. It will also provide tools and important information to help educate and support patients who are transitioning to a biosimilar.
Presenters from medSask and the Saskatchewan Drug Plan and Extended Benefits Branch recorded a webinar to review the use of Biosimilar drugs, and describe the Saskatchewan Biosimilars Initiative and the role of pharmacists and primary care providers in transitioning patients to biosimilar insulin.
Information about Biosimilars
- Key Points
- Biosimilars are safe and effective.
- Biosimilars are expected to work as well as reference biologics. No difference in adverse events or immunogenicity is expected.
- Biosimilars are closely regulated by Health Canada: There is a rigorous approval process that requires extensive comparative studies, and manufacturers must demonstrate high quality. Once authorized, manufacturers must also continue to monitor safety through post-marketing surveillance.
- Biosimilars have been used in Canada since 2009. Other provinces and territories in Canada and many countries around the world have implemented biosimilar policies.
- Research studies evaluating switching from a reference biologic to its biosimilar have found that switching is not associated with any major efficacy, safety, or immunogenicity issues.
Biologic Drug Resources
Health Canada Biosimilar Handbook for healthcare professionals offers a comprehensive review of biologic drugs and biosimilars and will give you foundational information to talk to individuals about the use of biosimilars.
The Health Canada Biosimilar biologic drugs in Canada: Fact Sheet explains key point about biosimilar drugs are and how they are regulated in Canada.
The Saskatchewan Biosimilars Initiative website provides key messaging to provide to patients about biosimilars as well as answers to questions that healthcare providers and patients impacted by the policy may be asking.
CADTH also offers quick answers to Frequently Asked Questions in a two page infographic for healthcare providers.
- Evidence
Links to Evidence: The Saskatchewan Biosimilars Initiative website has a PDF linking to important resources and evidence to support the use of biosimilars.
Switching to a Biosimilar
Two systematic reviews provide evidence on switching between reference biologics and biosimilars. Findings indicate that switching is not associated with any major efficacy, safety or immunogenicity issues.
- Switching Reference Medicines to Biosimilars: A Systematic Literature Review of Clinical Outcomes | SpringerLink
- The Efficacy, Safety, and Immunogenicity of Switching Between Reference Biopharmaceuticals and Biosimilars: A Systematic Review - PubMed (nih.gov)
Evidence from Other Jurisdictions:
British Columbia introduced a biosimilar policy in May 2019 and has published outcome data which can be found on the BC Biosimilars webpage.
Important Practice Points
- Interchangeability
- In Saskatchewan, biosimilars are not automatically interchangeable with the reference biologic.
- Biosimilars cannot be substituted in place of a reference biologic drug when a prescription for a particular biologic drug has been written.
- Prescriptions
- A new prescription is required for the patient to switch from a reference biologic to a biosimilar.
- Prescriptions must clearly indicate the biosimilar brand to be dispensed by the pharmacy.
- Pharmacies cannot substitute an alternative biologic drug when a prescription for a particular biologic drug has been written.
- Prescriptions for a reference biologic cannot be switched to a biosimilar version.
- Prescriptions for a specific biosimilar cannot be switched to an alternative biosimilar.
- Exception Drug Status (EDS)
- Where applicable, EDS coverage for the listed biosimilars has been added for patients who have current EDS coverage for an affected reference biologic. Their EDS coverage for the reference biologic will expire at the end of the announced transition period.
- Saskatchewan Biosimilars Initiative
- Patients who have all or a portion of the cost of their biologic drug paid by the Saskatchewan Drug Plan are required to transition to a biosimilar to maintain this coverage.
- Patients with drug coverage through federal programs such as NIHB, Veterans Affairs, and RCMP are not affected.
- Patients with third party drug coverage (e.g., Sun Life, Green Shield, Saskatchewan Blue Cross) through an employer or personal plan may not be affected. Patients should contact their plan to ask how the Saskatchewan Biosimilars Initiative may apply to their private plan, as third-party policies vary.
- Although patients may not need to transition to maintain coverage, this may be an opportunity to consider a transition to a biosimilar to support drug affordability.
Biosimilar Insulin and A-PAR
- Administrative Prescribing of Biosimilar Insulin
Like other biosimilar drugs in Saskatchewan, insulin biosimilars are not automatically interchangeable with the reference biologic. Individuals who need to transition to a biosimilar to maintain drug coverage under the Saskatchewan Drug Plan will require a new prescription.
If an individual is unable to otherwise obtain a new prescription for a biosimilar insulin from their usual diabetes care provider, pharmacists can help facilitate the transition. As a Schedule II drug, pharmacists have the authority to sell and recommend insulin. Per the Saskatchewan College of Pharmacy Professionals (SCPP) Administrative Prescribing policy, pharmacists may generate a prescription for a biosimilar insulin using their name as the prescriber. Pharmacists must follow the standards of practice for Schedule II drugs.
- It is necessary to establish that the initial need for insulin has already been identified by a primary care/specialist prescriber. Administrative prescribing is a continuation of therapy and not a new insulin start.
- Pharmacists are not permitted to take over management of the patient’s diabetes.
- If, while assessing the patient, concerns arise about the appropriateness of an insulin transition or diabetes management, consultation with the primary care/specialist prescriber may be warranted. It is important to maintain a safe supply of insulin.
- Pharmacists should not administratively prescribe insulin to themselves, family members, or persons with whom they have a close personal relationship. If a supply of insulin is provided in these situations, the circumstances and rationale should be documented.
medSask has developed tools to help support pharmacists in administrative prescribing of biosimilar insulins including a documentation and notification form (A-PAR), patient counselling infographic, and comparison charts of available insulins.
- Guide to Using the A-PAR
The Administrative Pharmacist Assessment Record (A-PAR) has been developed to standardize and document the process of collecting and assessing information to transition individuals to a biosimilar insulin.
A-PAR Biosimilar Insulin (fillable PDF)
- Transitioning from a reference biologic insulin to a biosimilar insulin is not likely to trigger loss of glycemic control or adverse effects. The A-PAR helps to assess current insulin therapy to ensure the safe transition to a biosimilar insulin.
- When reviewing a patient’s current insulin therapy, concerns about their diabetes management may be identified which a pharmacist may not be able to manage within their scope of practice. The A-PAR provides an opportunity to document these concerns and a plan to contact the primary care/specialist prescriber should the need arise.
- Use of the A-PAR is for the initial transition to a biosimilar insulin from a reference biologic insulin. Subsequent supply may be provided to the patient using administrative prescribing without completion of the A-PAR provided that the standards of practice for providing Schedule II drugs are met.
Note to Users: The electronic fillable A-PAR does not prevent the user from selecting more than one biosimilar version of a each reference insulin when completing the Product Selection section. Users of the form should take care that only one biosimilar insulin format per reference biologic insulin is selected. (i.e.: When transitioning from Lantus®, only one of Basaglar® cartridge, Basaglar® prefilled pen, or Semglee® prefilled pen should be selected.)
PRODUCT SELECTION
Review previous insulin use and collaborate with the patient to determine the preferred biosimilar insulin and delivery device to transition to.
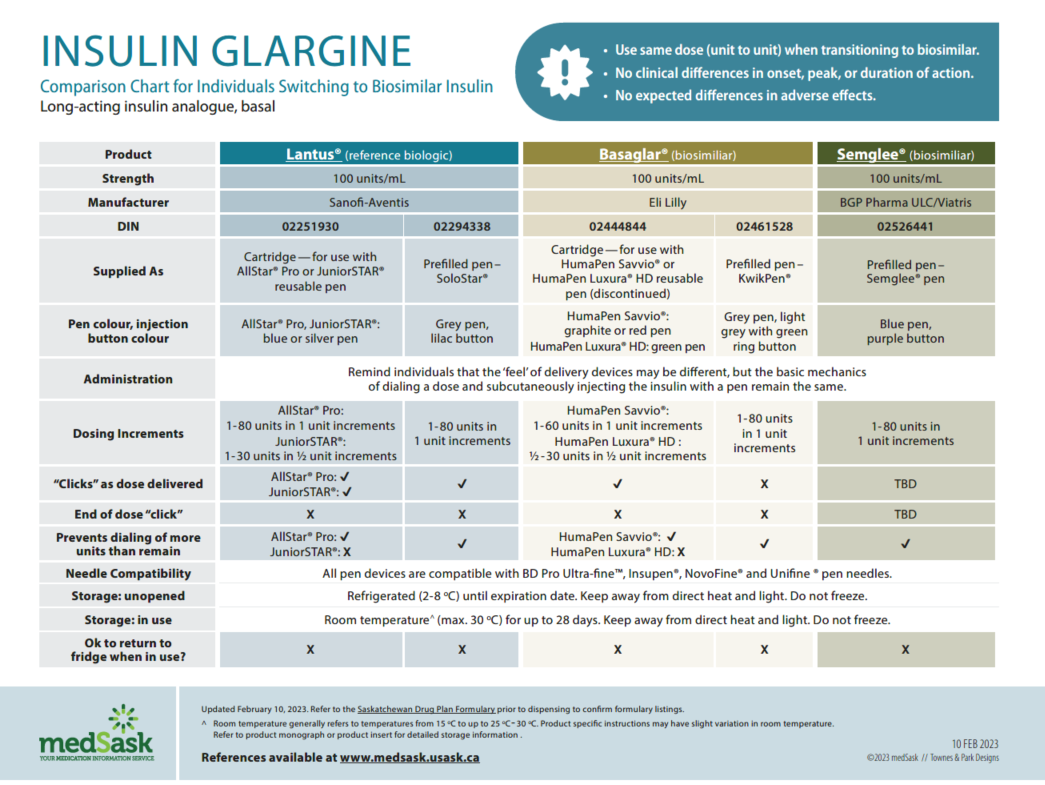
Insulin Glargine Comparison Chart
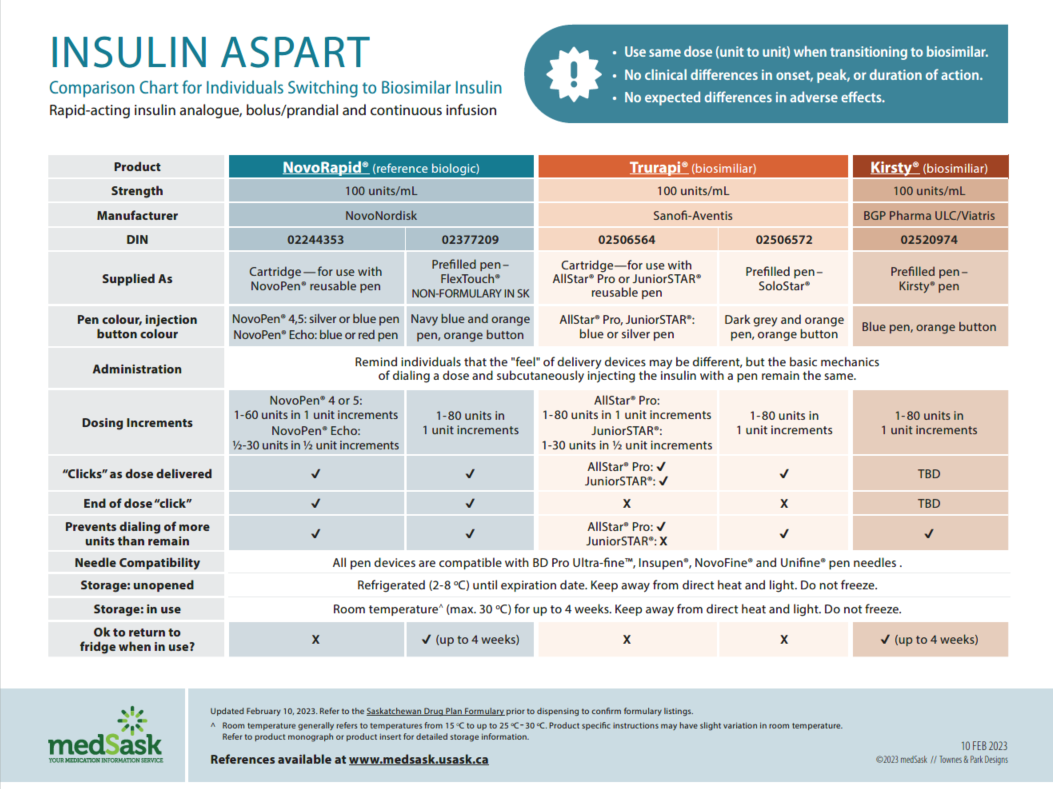
Insulin Aspart Comparison Chart
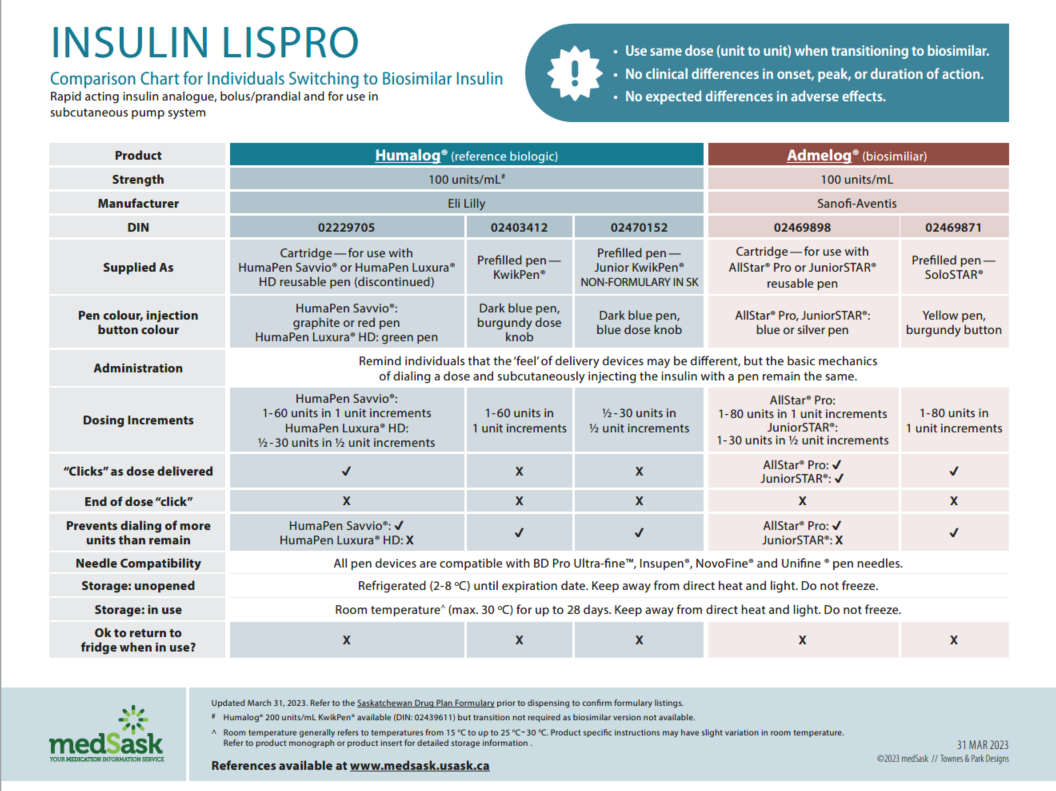
Insulin Lispro Comparison Chart
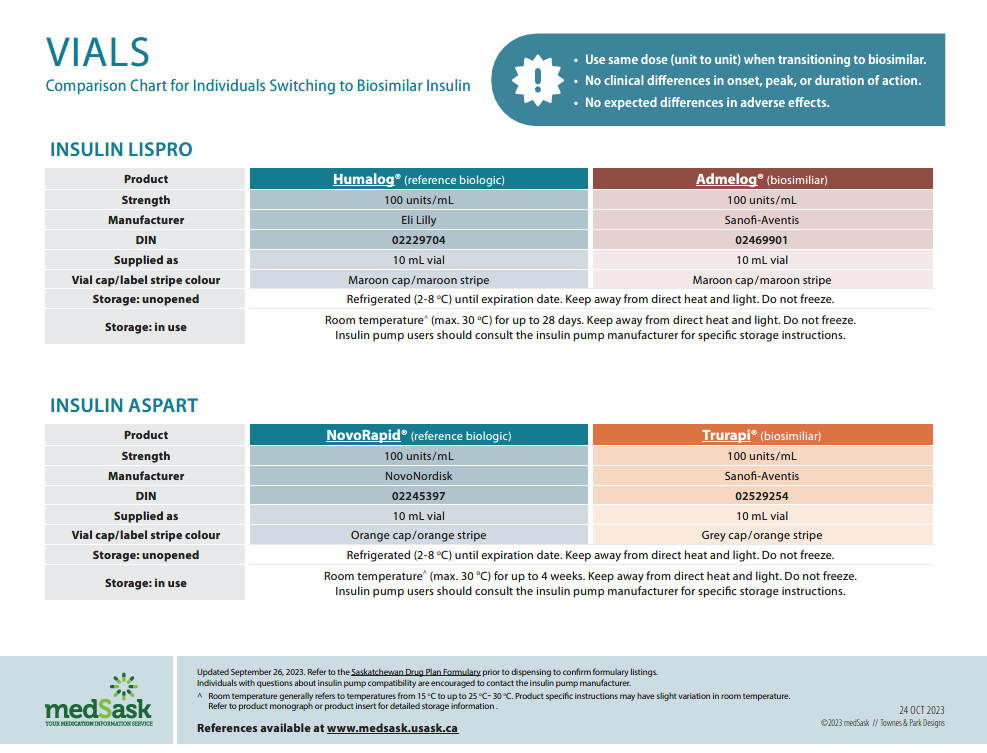
Insulin Vials Comparison Chart
- There are no differences in efficacy or safety amongst the reference and biosimilar insulins. Use the medSask Insulin Comparison Charts to assist with product selection.
- Confirm the previous insulin directions as per Pharmaceutical Information Program (PIP)/eHealth, the patient/designate, and/or the pharmacy patient record.
- The dose and administration time(s) for biosimilar insulin remain the same as the reference biologic insulin. “Take as directed” is acceptable as directions.
- It may be prudent to make one transition at a time rather than introducing two new biosimilars at once.
MEDICAL INFORMATION
Confirm prior initiation of and review current insulin therapy via patient with review of PIP/eHealth as appropriate.
- HbA1C: If the patient has not had routine monitoring of A1C or if the A1C exceeds the target, transition them to a biosimilar and refer them to primary care/specialist provider or Certified Diabetes Educator (CDE).
- Review of current diabetes management allows the pharmacist to confirm that a transition to biosimilar insulin is safe and appropriate, and to identify issues related to insulin therapy that may require referral to the primary care/specialist prescriber or a CDE.
- Refer to Diabetes Canada Guidelines for review of recommended targets and diabetes management.
ASSESSMENT AND PLAN
Document relevant information and any concerns about insulin therapy, and indicate the action taken.
- Patients should follow up with their primary care/specialist prescriber following a transition to a biosimilar insulin. Diabetes Canada guidelines suggest to have HbA1C measured every 3 months when insulin therapy is adjusted. Recommend that patients schedule an appointment within 3 months (or sooner if appropriate) to have insulin therapy reviewed.
- For concerns related to current diabetes management beyond the scope of a pharmacist, refer the patient to the primary care/specialist prescriber and/or a CDE.
- Use the Find a CDE tool to locate a CDE in your community.
- Saskatchewan Health Authority Primary Care clinics throughout the province have diabetes education teams including CDEs, nurses, dietitians, and pharmacists. Contact your local primary health care network office to locate your specific diabetes education team and referral processes.
- Patients without a primary care provider can use the Physician Search on the College of Physicians and Surgeons of Saskatchewan website to find a list of physicians in their area.
ADMINISTRATIVE PRESCRIBING OF BIOSIMLAR INSULIN
- Documentation of obtaining informed consent from the patient is required. Patients/designates have the right to be informed about the benefits and risks of transitioning to a biosimilar insulin, have an opportunity to ask questions, and make a voluntary decision about transitioning. Consent includes permission to access PIP/eHealth viewer and notify other care providers.
- All prescribed medication (including Schedule II, III, and unscheduled) must be entered into the patient’s PIP profile as per the Prescription Drugs Act. (See SCPP PIP FAQs).
- The A-PAR must be retained as per SCPP Record Keeping Requirements.
PATIENT EDUCATION
Counselling points are noted on the A-PAR.
- The Insulin Comparison Charts can be used to ensure patient is aware of similarities and differences between their previous insulin and the biosimilar insulin.
- The Transitioning to a Biosimilar Insulin infographic can be provided to patients as a reminder of key education points. The insulin regimen section is to be completed by the pharmacist.
- Ensure the patient has the correct pen for the cartridge they are using and necessary supplies (e.g. pen needles, glucose testing strips, lancets, glucose/glucagon).
- Encourage patients to return any remaining supply of reference biologic insulin(s) and previous pen device(s) to the pharmacy for disposal prior to starting the biosimilar insulin to prevent confusion and subsequent administration errors.
FOLLOW-UP
Follow-up with the patient after transitioning to biosimilar insulin.
- No differences in glycemic control, hypoglycemia, and adverse events are expected with the biosimilar insulin. If a patient reports an issue with the transition, loss of glycemic control, or an adverse event or loss of glycemic control, the pharmacist can assist as appropriate or refer the patient to the primary care/specialist prescriber, CDE, or urgent/walk-in care.
PROVIDER NOTIFICATION
Notify the primary care/specialist prescriber about the transition to a biosimilar or indicate if patient does not have primary care provider using the Provider Notification Form that follows the A-PAR.
- Products
Please see Saskatchewan Biosimilars website for current insulins affected, available biosimilars, and important exceptions to the list of insulins affected.
Reference Biologic InsulinBiosimilar InsulinExceptions and Notes:
Aspart
NovoRapid®- Cartridge
- FlexTouch® prefilled pen
- Vial
Trurapi®
- Cartridge
- Solostar® prefilled pen
Kirsty®
- Prefilled pen
- NovoRapid vials will remain covered for existing users until biosimilar(s) in a vial format are listed on the Saskatchewan Formulary.
- Coverage of NovoRapid will continue to be available for patients who use insulin pumps while the biosimilars undergo insulin pump certification.
- Individuals who use vial may be transitioned to a biosimilar insulin in a cartridge/prefilled pen to support patient care/preference.
- NovoRapid® FlexTouch® prefilled pens are not on Saskatchewan Formulary, however, patients may be transitioned to biosimilar insulin to support patient care/preference.
- Trurapi® vials are available, but not on the Saskatchewan Formulary.
- Kirsty® vials are not yet marketed and not on the Saskatchewan Formulary.
Glargine
Lantus®
- Cartridge
- Solostar® prefilled pen
- Vial
Basaglar®
- Cartridge
- Kwikpen® prefilled pen
Semglee®
- Prefilled pen
- Lantus® vials will remain covered for existing users until biosimilar(s) in a vial format are listed on the Saskatchewan Formulary.
- Individuals who use vial may be transitioned to a biosimilar insulin in a cartridge/prefilled pen to support patient care/preference.
- Prescribers may apply for EDS coverage for pediatric patients who require ½ unit device to administer insulin glargine.
Lispro
Humalog®
- Cartridge
- Kwikpen® prefilled pen
- Junior Kwikpen® prefilled pen
- Vial
Admelog®
- Cartridge
- Solostar® prefilled pen
- Vial
This policy applies to all Humalog® 100 units/mL products including the Humalog® Junior Kwikpen and the vial format.
Admelog is compatible with various insulin pump models from Insulet (Omnipod), Medtronic, Tandem, and Ypsomed
Individuals with questions about insulin compatibility with specific insulin pump models are encouraged to contact the insulin pump manufacturer.
Individuals using Humalog® 200 unit/mL insulin do not need to transition at this time, since there is no biosimilar version available.
Product chart updated March 19, 2024.
- Prescribing and Billing Details
May administratively prescribe sufficient quantity to ensure insulin therapy is not interrupted. Quantity is determined at the discretion of the pharmacist to ensure insulin therapy is not interrupted. Administrative prescribing does not replace the need for follow-up with primary care/specialist prescriber for ongoing diabetes management.
A Biosimilar Insulin Transition Fee (BITF) will be paid to pharmacies to assist in the transition to biosimilar insulin. The BITF Policy and Billing Procedure is posted on the secure Drug Plan Web page for pharmacies and under Resources and Studies on the Saskatchewan Biosimilars Initiative website.
- Additional Resources
medSask Key Point Infographics
- medSask Biosimilar Patient Communication - Admelog
- medSask Biosimilar Patient Communication - Basaglar and Semglee
- medSask Biosimilar Patient Communication - Trurapi and Kirsty
medSask Practice Tools
- Biosimilar Insulin Safety Labels - Humalog
- Biosimilar Insulin Safety Labels - Lantus
- Biosimilar Insulin Safety Labels - NovoRapid
General Information
- Basaglar® Product Monograph
- Kirsty® Product Monograph
- Semglee® Product Monograph
- Trurapi® Product Monograph
- Admelog® Product Monograph
Insulin Pump Manufacturers
Adverse Reaction Reporting
- Health Canada - https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-reporting/drug.html
Diabetes Guidelines
Support for Indigenous people
Transitioning to a Biosimilar
- The Saskatchewan Biosimilars Initiative Policy
The Saskatchewan Biosimilars Initiative policy can be found here.
Frequently Asked Questions for patients and healthcare providers, including information about exception drug status and medical exemptions can be found here.
- Information and Education for Patients
General information about biosimilars
- medSask infographic
- Health Canada Biosimilar Fact Sheet
- CADTH Biosimilar Drugs: Your Questions Answered
- Making the Most of Your Biosimilar
Condition specific information about biosimilars
- Arthritis Society: Concerned about switching to a biosimilar medication?
- Canadian Digestive Health Foundation Biosimilar Library - resources for Inflammatory Bowel Disease (IBD) patients to understand the process of switching to a biosimilar
- Arthritis Society Canada Biologics and Biosimilars for the Treatment of Inflammatory Arthritis – answers to general questions about biosimilars and specific information about biosimilar for Inflammatory Arthritis
- Joint Health Biosimilars Exchange – general information about biosimilars from a patient-led organization
Multiple sclerosis (MS Canada): Generic, biosimilar and subsequent entry non-biologic complex drugs
- Patient Support Programs
The Saskatchewan Biosimilars Initiative website has a directory of the Patient Support Programs and provides contact information and enrollment instructions.
- The Patient Support Program (PSP) will change when patients transition from a reference biologic to a biosimilar.
- Patients will need to be enrolled in the PSP specific to the biosimilar that they transition to. Enrollment is usually done by prescribers and/or patients.
- PSPs are an important resource that people using a biologic drug can access. Timely enrollment is important to ensure ongoing support.
- Patients who have not previously accessed the services of a PSP may be interested in the tools that are available to help facilitate a transition.
- Follow-Up/Adverse Event Reporting
Follow Up
It is important to check in with patients after they have transitioned to a biosimilar. Follow-up provides an opportunity to discuss efficacy, ask about adverse effects, and to give the patient chance to ask questions.
Follow up may include:
- Administration
- Correct use of delivery device
- Coordination with infusion clinic as appropriate
- Control of the condition/disease being treated and symptom management
- Loss of efficacy is not likely to be triggered by a switch to a biosimilar
- Adverse effects
- Adverse events are not likely to be different from those experienced from the reference biologic.
- Should an adverse event occur following a switch it should be reported to Health Canada.
- For pharmacists: Refer the patient to the prescriber if the adverse event could be attributed to the biosimilar switch.
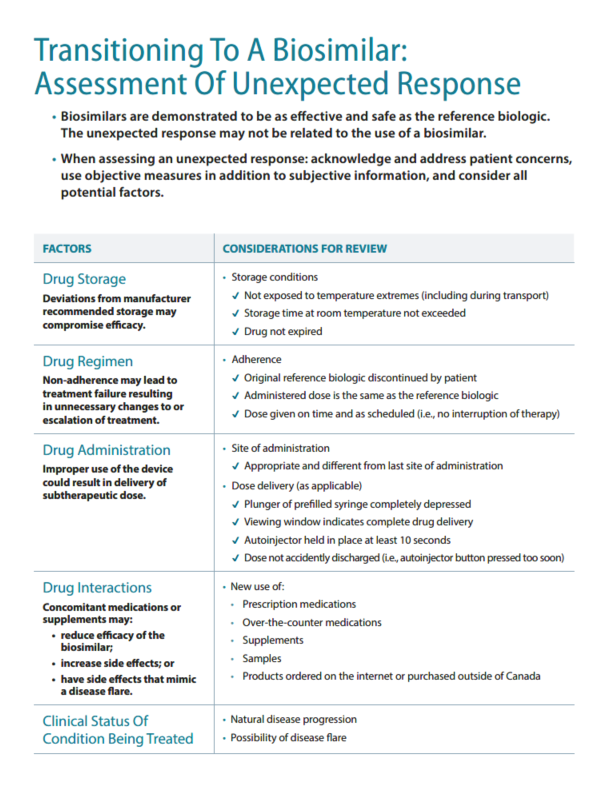
Assessment of Unexpected Response
Reporting Adverse Events
Healthcare professionals, including pharmacists, play an important role in pharmacovigilance of biosimilars. It is important to report observations of patient responses for ongoing understanding of the drug safety profile.
When reporting an adverse reaction to a biosimilar, it is important to include the brand of the biosimilar product (and lot number if possible) and indicate whether the patient has initiated treatment with the biosimilar, is switching from a reference biologic drug, or is switching from one biosimilar to another.
Adverse events are reported to Health Canada here: Health Canada Adverse Event Reporting
Exemptions
Prescribers can request an exemption to maintain coverage of the reference biologic if their patient cannot use a biosimilar for a medical reason. Refer to the Saskatchewan Biosimilars Initiative website for information and to access Prescriber Forms: Saskatchewan Biosimilars Initiative | Extended Benefits and Drug Plan | Government of Saskatchewan
- Administration
References
- References
- Saskatchewan Health. Saskatchewan Biosimilars Initiative. Regina: Government of Saskatchewan; [cited 07 Nov 2022]. Available from Saskatchewan Biosimilars Initiative | Extended Benefits and Drug Plan | Government of Saskatchewan
- Saskatchewan Drug Plan. Saskatchewan Online Formulary Database. Regina: Government of Saskatchewan; [cited 19 Dec 2022]. Available from: http://formulary.drugplan.health.gov.sk.ca/SearchFormulary
- Health Canada. Biosimilar biologic drugs in Canada. Ottawa, ON: Health Canada; [Updated 2019 Aug 27; cited 19 Dec 2022] Available from: Biosimilar biologic drugs in Canada: Fact Sheet - Canada.ca
- Health Canada. Consultation: Handbook for healthcare professionals on biosimilar biologic drugs in Canada. Ottawa, ON: Health Canada; [updated 29 April 2022; cited 16 Jan 2023]. Available from: Consultation: Handbook for healthcare professionals on biosimilar biologic drugs - Canada.ca
- Health Canada. Guidance Document: Information and Submission Requirements for Biosimilar Biologic Drugs. Ottawa, ON: Health Canada; [Updated 2022 Aug 26; cited 16 Jan 2023]. Available from: Guidance Document: Information and Submission Requirements for Biosimilar Biologic Drugs - Canada.ca
- Health Canada. Drug Product Database Online Query. Ottawa, ON: Health Canada; [updated 2022 Mar 10; cited 2022 Dec 12]. Available from: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp